Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Miyajima, Yusuke*; Saito, Ayaka*; Kagi, Hiroyuki*; Yokoyama, Tatsunori; Hirata, Takafumi*
no journal, ,
Calcium carbonates are ubiquitously present throughout the Earth history as animal shells, speleothems, fault-related vein fillings, and hydrothermal or cold-seep precipitates. Direct dating of carbonates provides valuable information on paleoenvironmental change, tectonics, and fluid and material cycling. U-Pb dating using high spatial-resolution LA-ICP-MS is a key technique to date natural carbonates. In situ U-Pb dating by LA-ICP-MS needs matrix-matched reference materials to correct matrix-dependent elemental fractionation in LA-ICP-MS. Roberts et al. (2017) demonstrated that a natural calcite cement WC-1 is suitable as a calcite reference material. However, the WC-1 calcite has an inhomogeneous distribution of U and Pb and lacks concordance in the U-Pb system. In this study, we synthesized novel calcite reference materials with homogeneous U and Pb concentrations and isotope ratios. We incorporated U and Pb into calcite through heat-induced crystallization from U, Pb-doped amorphous calcium carbonate (ACC). The homogeneity of the U/Ca and Pb/Ca ratios in the synthetic calcite was generally better than 8% and 13%, respectively, in relative standard deviation. The 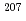 Pb/
Pb/ Pb ratio of the synthetic calcite was homogeneous within ~1% errors, whereas the
Pb ratio of the synthetic calcite was homogeneous within ~1% errors, whereas the  U/
U/ Pb ratio was less homogeneous (3%-11% errors). To test the usability of the synthetic calcite, we dated WC-1 using the synthetic calcite for correction of elemental fractionation. We calculated the nominal
Pb ratio was less homogeneous (3%-11% errors). To test the usability of the synthetic calcite, we dated WC-1 using the synthetic calcite for correction of elemental fractionation. We calculated the nominal  U/
U/ Pb ratio in the synthetic calcite from its U and Pb concentrations. We then obtained the fractionation factor to correct bias between the isotope ratios and the nominal value. We could accurately date WC-1 with an ~3% precision. If the isotopic compositions of the synthetic calcite are certified by isotope-dilution technique, we could date natural carbonates with
Pb ratio in the synthetic calcite from its U and Pb concentrations. We then obtained the fractionation factor to correct bias between the isotope ratios and the nominal value. We could accurately date WC-1 with an ~3% precision. If the isotopic compositions of the synthetic calcite are certified by isotope-dilution technique, we could date natural carbonates with  10% precisions using the synthetic reference calcite.
10% precisions using the synthetic reference calcite.